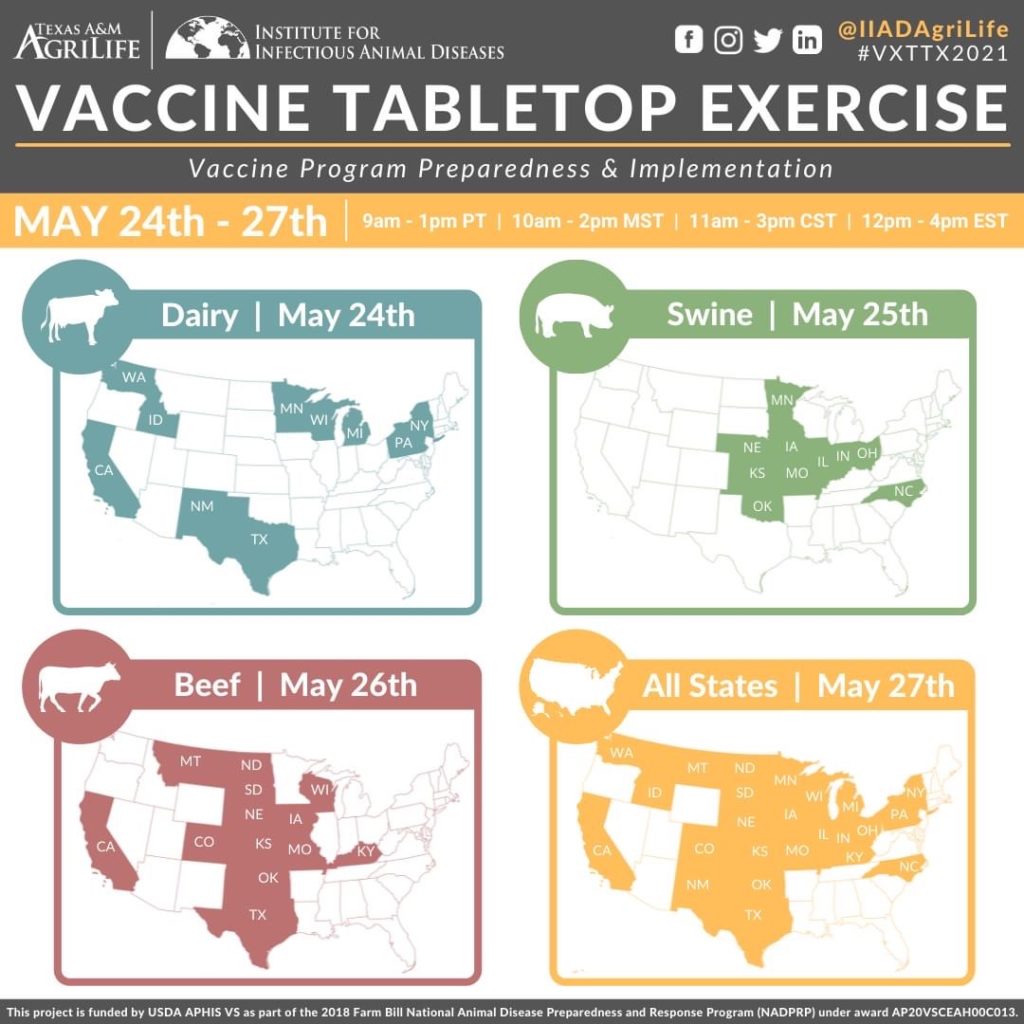
Featured Projects, Tools and Educational Opportunities
Our Global Impact
The Institute for Infectious Animal Diseases is recognized by the World Organization for Animal Health (WOAH - formerly known as OIE) as a collaborating center in the specialty of biological threat reduction. As a collaborating center, IIAD provides its expertise internationally to support and implement animal health initiatives, provide scientific and technical training, and conduct scientific research focused on global animal health. We are the only biological threat reduction center in the WOAH’s America’s region and the only WOAH collaborating center within the Texas A&M University System.